X-Chromosome Inactivation: A Breakthrough in Genetics
X-chromosome inactivation is a remarkable biological process that plays a pivotal role in cellular function, especially in females who possess two X chromosomes. This intricate mechanism ensures that females do not express double the amount of genes carried on the X chromosome compared to males, who only have one copy. A team from Harvard, led by Jeannie T. Lee, has been at the forefront of investigating this complex phenomenon, revealing potential applications for treating conditions like Fragile X Syndrome and Rett Syndrome through innovative gene silencing breakthroughs. Their research highlights how the Xist RNA function interacts with cellular structures, akin to a Jell-O-like substance, to successfully silence one of the X chromosomes, thus opening pathways to therapies. With insights gained from decades of study, this work not only enhances our understanding of genetics but also paves the way for transformative treatments aimed at alleviating the suffering caused by genetic disorders linked to the X chromosome.
The process referred to as X chromosome inactivation is critical for gene regulation in human cells, particularly in females, where it serves to balance gene dosage between the sexes. Known by various terms, this cellular mechanism allows one of the X chromosomes in females to be silenced, ensuring that they do not produce an excess of X-linked gene products. Research spearheaded by experts at Harvard has delved into this phenomenon, identifying the role of Xist RNA and a gelatinous matrix that enables effective gene silencing. Such discoveries are vital, especially in relation to genetic disorders like Fragile X Syndrome and Rett Syndrome, where targeting the inactive X chromosome could lead to effective therapies. As researchers explore the potential of gene therapy, understanding this regulatory process becomes crucial for developing targeted treatments that can bring hope to those affected by these conditions.
The Significance of X-Chromosome Inactivation
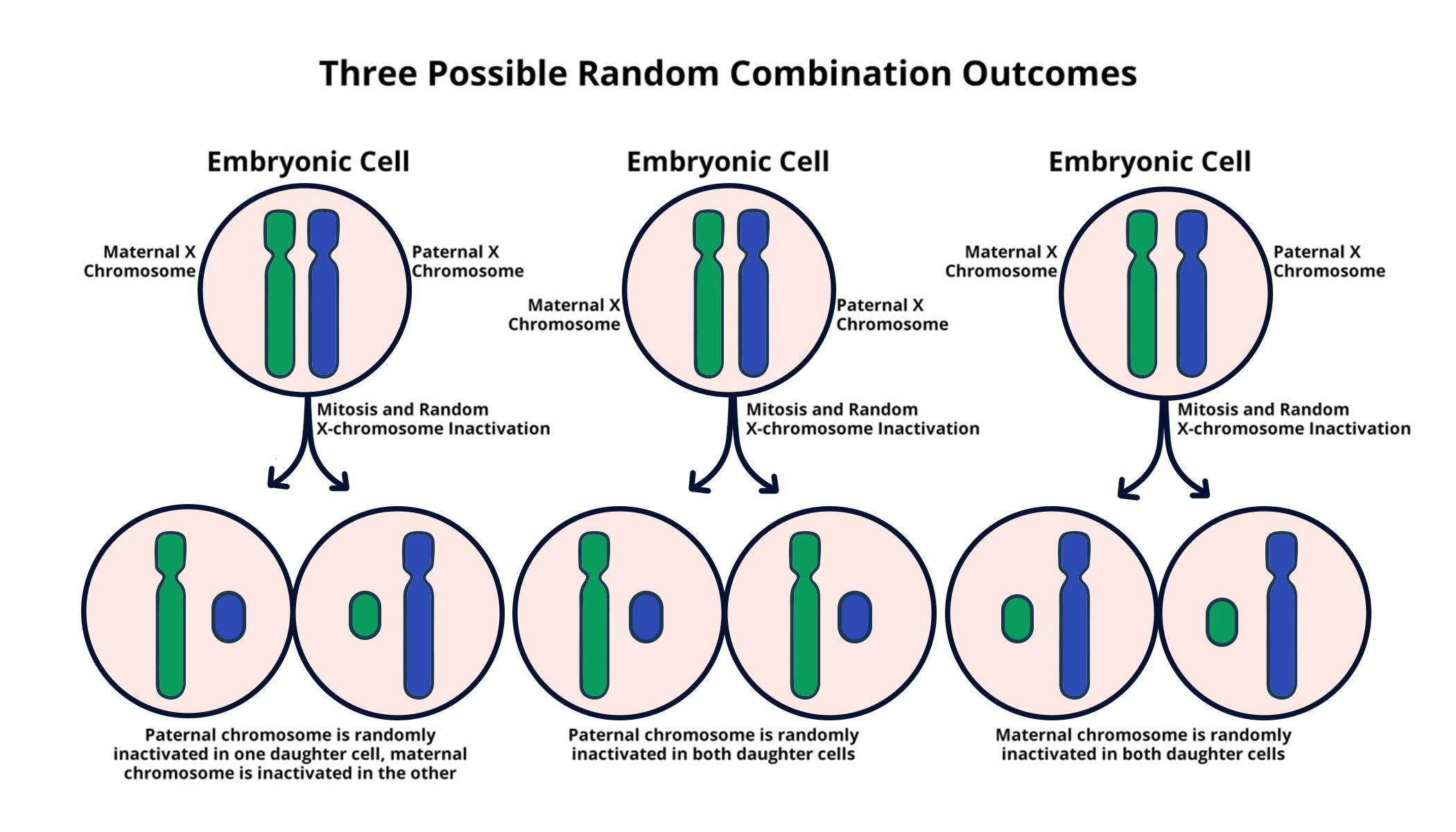
X-chromosome inactivation (XCI) is a critical biological process that ensures dosage compensation between males and females for genes located on the X chromosome. In females, where there are two X chromosomes, cellular mechanisms must silence one copy to prevent an overabundance of gene products. This biological balancing act is essential for maintaining normal cellular function and preventing conditions that may arise from gene dosage imbalances. The discovery of how this intricate mechanism operates not only enhances our knowledge of genetics but also opens doors to potential therapies for X-linked disorders.
Recent research elucidates that the Xist RNA plays a pivotal role in initiating X-inactivation. By binding to the X chromosome and modifying the surrounding chromatin structure, Xist effectively makes the chromosome inert. Understanding the precise mechanics of XCI has far-reaching implications for diseases like Fragile X Syndrome and Rett Syndrome, which are caused by mutations on the X chromosome. If scientists can manipulate or reverse this inactivation process, as current studies by the Lee lab aim to do, there may be groundbreaking treatment options available in the near future.
Breakthroughs in Gene Silencing
Recent advances in gene silencing techniques present a fascinating frontier in genetic research. Breakthroughs in the understanding of X-chromosome inactivation have given rise to innovative approaches to unsilencing genes associated with conditions such as Fragile X Syndrome. These treatments seek to restore the function of healthy genes that are typically inaccessible due to the inactivated state of one of the X chromosomes in females. The therapeutic potential is immense, not just for women, but also for males, who may suffer from conditions arising from mutations in their single X chromosome.
One notable strategy involves the use of Xist RNA to target specific inactivated X chromosomes, thereby facilitating the reactivation of crucial genes. The research led by Jeannie Lee at Harvard underscores the promise of this approach, which could redefine treatment paradigms for genetically linked diseases. As researchers continue to explore the potentials of Xist and other molecular players in chromosomal silencing, the prospect of developing effective therapies becomes increasingly tangible. These advancements may significantly alter the landscape of genetic disorders treatment in the coming years.
Fragile X Syndrome Treatment Potential
Fragile X Syndrome is caused by a mutation in the FMR1 gene located on the X chromosome, leading to intellectual disability and developmental challenges. Current treatment options are primarily symptomatic, focusing on managing behavioral and emotional problems rather than addressing the underlying genetic cause. However, recent promising research led by experts like Jeannie T. Lee suggests that by employing methods to unsilence the inactivated X chromosome, it may be possible to activate the unaffected allele, potentially curing the disorder. This would mark a significant advancement in the treatment of genetic diseases.
As Lee’s studies continue to evolve, the hope is that de-silencing techniques could translate into viable therapies for Fragile X Syndrome. The deployment of gene silencing breakthroughs derived from extensive Harvard genetics research showcases the potential of not just managing symptoms but reversing the effects of genetic mutations. With safety studies in the pipeline, there’s optimism about moving these innovative treatments into clinical trials, which could herald a new era in the fight against X-linked genetic disorders.
Exploring Rett Syndrome Therapy Innovations
Rett Syndrome, another neurodevelopmental disorder linked to mutations on the X chromosome, also stands to benefit from these innovative research efforts. Much like Fragile X Syndrome, the affected individuals often have a mutated gene that compromises their neurological development. Researchers are excited about the implications of using the Xist RNA mechanism to reactivate the silenced gene, potentially restoring normal function. This gene-silencing breakthrough represents a fuller understanding of Rett Syndrome and how therapeutic strategies can be implemented effectively.
By utilizing the insights gleaned from studies of X-chromosome inactivation, scientists may develop targeted therapies for Rett Syndrome that address the root causes rather than merely alleviating symptoms. The goal is to bring forth practical solutions that can significantly improve the quality of life for those affected. The pathway to such treatments involves rigorous research and connection to pioneering efforts at institutes like Harvard, which continue to shape our understanding of X-linked disorders.
The Role of Harvard Genetics Research in Advancements
Harvard University has been at the forefront of genetics research, contributing significantly to our understanding of fundamental processes like X-chromosome inactivation. The work of Jeannie T. Lee and her team exemplifies the innovative spirit of Harvard’s research community, which is not only focused on basic science but also on translational research that can lead to tangible therapeutic outcomes. Their dedication to answering complex biological questions is paving the way for breakthroughs that are expected to revolutionize the treatment landscape for genetic disorders.
The culmination of decades of research has unfurled strategies such as manipulating Xist RNA to combat diseases like Fragile X and Rett Syndromes. This ongoing work reflects the symbiotic relationship between basic research and clinical applications, highlighting how insights gained from years of lab exploration translate into real-world therapies. As researchers at institutions like Harvard continue to push the boundaries of genetic science, the future of treatment for X-linked disorders grows brighter.
Understanding Xist RNA Function
Xist RNA plays a fundamental role in X-chromosome inactivation, acting as a key regulator in the silencing process. This fascinating RNA molecule coats the inactivated X chromosome, altering its three-dimensional structure and rendering it inactive. The mechanics of how Xist engages with the chromatin matrix—akin to the gelatinous ‘Jell-O’ described by researchers—demonstrate how complex and finely tuned this regulatory system is. Understanding Xist’s function is crucial for developing targeted therapies for X-linked diseases, as it represents both a challenge and an opportunity in genetic research.
The ongoing exploration of Xist’s role has led to the hypothesis that manipulating this RNA could lead to groundbreaking interventions for conditions caused by X-linked mutations. For example, if scientists can find ways to control or alter the activity of Xist, they may unlock the potential to reactivate silenced genes, providing a pathway for effective treatments for Fragile X Syndrome and Rett Syndrome. This research not only sheds light on the biology of X-inactivation but also propels forward the therapeutic possibilities that can derive from this process.
Navigating the Safety Studies for Gene-Bridging Techniques
As promising as the scientific advances may seem, safety remains a paramount concern in the journey toward potential therapies for X-linked genetic disorders. As Jeannie T. Lee’s lab prepares to conduct safety studies, researchers are diligently assessing the implications of unsilencing X-linked genes. While the initial findings are hopeful—that de-silencing techniques might target diseased genes without affecting healthy ones—comprehensive evaluations are essential to ensure that these interventions do not introduce unforeseen risks.
Establishing safety protocols is a critical step before moving to human clinical trials, particularly in the context of complicated genetic conditions like Fragile X and Rett Syndromes. The collaborative effort between geneticists and clinical researchers at institutions like Harvard is integral in shaping these safety studies, ensuring that the transition from laboratory work to practical treatments is executed with the greatest caution. The ongoing dialogue about the efficacy and safety of these innovative therapies reflects the complexity of medical advancement in genetics.
The Future of Gene Therapy: Moving Toward Clinical Trials
The future of gene therapy, particularly in the context of disorders linked to the X chromosome, is gaining momentum as foundations laid by recent research materialize into actionable clinical strategies. As the work of researchers like Jeannie T. Lee progresses towards clinical trials, the anticipation builds around the innovative treatment options that may soon emerge. With a strong focus on delivering solutions for conditions such as Fragile X Syndrome and Rett Syndrome, the paradigm for treating genetic disorders is rapidly evolving.
The translation of laboratory breakthroughs into clinical applications represents a significant leap for genetic medicine. As researchers refine techniques for X-linked gene activation and undertake necessary safety assessments, the possibility of successful therapy for patients afflicted by devastating genetic conditions becomes increasingly feasible. The collaboration of leading geneticists and commitment to scientific inquiry positions us at the brink of a new era in the treatment of X-linked genetic disorders, illuminating hope for many affected individuals.
Frequently Asked Questions
What is X-chromosome inactivation and how does it relate to gene silencing breakthroughs?
X-chromosome inactivation is a biological process in which one of the two X chromosomes in females is silenced to prevent the overexpression of X-linked genes. This gene silencing is essential for normal development and is key in research focusing on diseases caused by mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome. Recent breakthroughs in understanding the mechanisms behind Xist RNA function in X-inactivation have opened new therapeutic pathways for these genetic disorders.
How does Xist RNA function contribute to the treatment of Fragile X Syndrome?
Xist RNA plays a critical role in X-chromosome inactivation by modifying the biophysical properties of surrounding chromosomal structures. By understanding Xist RNA function, researchers have developed strategies to unsilence genes on the inactivated X chromosome, potentially leading to treatments for Fragile X Syndrome. These approaches aim to make the healthy version of genes accessible for cellular function, offering hope to those affected by the disorder.
What role does Harvard genetics research play in understanding X-chromosome inactivation?
Harvard genetics research, particularly from Jeannie T. Lee’s lab, has been pivotal in unraveling the complexities of X-chromosome inactivation. Their studies have identified the mechanisms by which Xist RNA alters chromosomal interactions, paving the way for innovative therapies for genetic disorders like Fragile X Syndrome and Rett Syndrome through targeted gene silencing.
Can X-chromosome inactivation therapies benefit males with X-linked mutations?
Yes, while males typically have only one X chromosome and do not undergo X-chromosome inactivation, similar mechanisms can silence mutated genes on their single X chromosome. Therapeutic strategies derived from understanding X-chromosome inactivation could potentially lead to effective treatments for males affected by conditions such as Fragile X Syndrome, by targeting and restoring the expression of healthy genes.
What are the future implications of X-chromosome inactivation research for treating genetic disorders?
Research into X-chromosome inactivation holds significant promise for treating genetic disorders like Fragile X Syndrome and Rett Syndrome. By unlocking the potential to unsilence inactivated X chromosomes, therapies can aim to restore the function of healthy genes, providing a more targeted approach with minimal side effects. As findings from Harvard universities and other research institutes progress, clinical trials are anticipated in the coming years, potentially transforming treatment options for patients.
| Key Aspects | Details |
|---|---|
| X-Chromosome Inactivation | Females have two X chromosomes but only need one active copy, hence one is inactivated. |
| Role of Xist | Xist RNA changes the properties of the gelatinous material surrounding the X chromosome, leading to its inactivation. |
| Potential Treatments | Understanding X-chromosome inactivation may lead to therapies for Fragile X and Rett syndromes. |
| Importance of the Jell-O-like Substance | This substance creates separation around chromosomes to prevent tangling and facilitates the process of inactivation. |
| Clinical Development | Research is ongoing, with hopes to move towards clinical trials for new treatments. |
Summary
X-chromosome inactivation is a crucial biological process that allows female cells to effectively manage their two X chromosomes by inactivating one. This innovative research led by Jeannie T. Lee reveals how this process can potentially pave the way for new treatments for genetic disorders linked to the X chromosome, such as Fragile X and Rett syndromes. The findings demonstrate how advances in understanding cellular mechanisms can lead to significant medical breakthroughs.